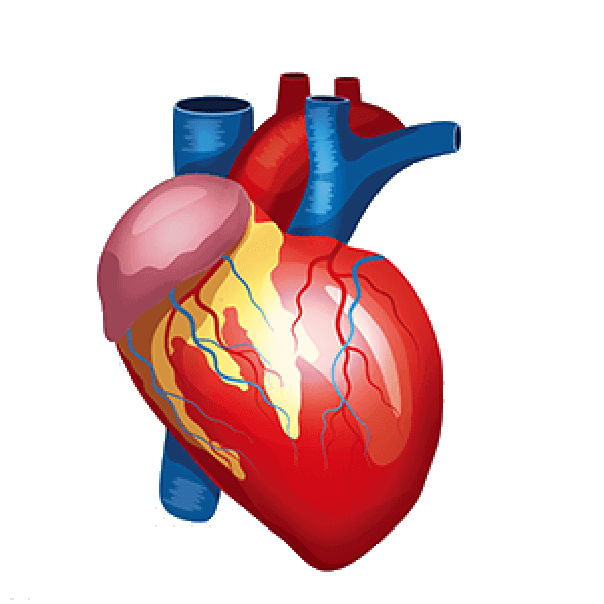
Device A defect or opening between the right and left sides of the heart is closed by a closure. Four chambers make up the heart. Atria refers to the upper two chambers. The Atrial Septum, a wall that divides the atria, is present. Ventricles-Right and Left are the names of the bottom chambers. The Left Atrium then gets oxygenated blood from the pulmonary veins. To circulate oxygenated blood throughout the body, the left atrium delivers this blood to the left ventricle via the mitral valve.
The heart's well-separated walls support a healthy circulatory system. However, in some congenital heart defect circumstances, any holes on the heart—whether in the atrial wall or the ventricle wall—can cause disrupted blood circulation and heart failure. If the holes are quite small, they may be surgically filled with some metal components. DEVICE CLOSURE is the name given to this idea. Therefore, in Device Closure, a metal device is surgically inserted into the location where a hole exists in a heart in order to retain the heart's functionality.
To close a hole or defect between the right and left sides of the heart, closure devices are employed. Some of these birth abnormalities are found in the septum, which separates the heart's upper chambers (atria):
Using a unique closure device, PFO and ASD are percutaneously closed. A special catheter, like the one used during your catheterization, is used to fold or attach the device. The catheter is placed into a vein in the leg, moved through the defect in the heart, and then removed. Each side of the device opens up and covers each side of the hole (like a sandwich), closing the hole or defect, as it is gradually pushed out of the catheter. The gadget is released from the unique catheter after it is in the ideal place. Heart tissue eventually grows over the implant and fuses with the heart.
The identification of the right patient is a crucial first step in a successful treatment. The procedure in the catheterization lab can be outlined step-by-step as follows: (I) hemodynamic study and evaluation of the morphologic characteristics of the defect; (II) establishment of the procedural strategy, including the equipment to be used and the procedure-guiding modality; (III) selection of the ideal type and size of the device; (IV) device implantation with warnings for potential complications such as air embolism and damage to cardiac/vascular structures; and (V) post-implantation assessment. The right patient education and follow-up are also crucial components of the treatment after a successful procedure. Each phase of the procedure's specifics and particular considerations have been well-described in the past. Individual topics, like procedure-guiding modalities, measuring the defect, and sealing complex defects, are frequently contested.
Issues with imaging guidance and defect sizing
It is commonly advised to carry out the procedure while under both fluoroscopic and echocardiographic supervision, despite studies reporting device closure guided exclusively by fluoroscopy or echocardiography. Transesophageal echocardiography (TEE) has long been the accepted modality for ASD closure for echocardiographic guidance. However, in recent years, intracardiac echocardiography (ICE) has been steadily taking over the function of TEE. Additionally, transthoracic echocardiography (TTE) may be performed, particularly in patients with favorable echocardiographic windows, such as young children.
The relevance of balloon sizing to the choice of device size has been up for debate. Although balloon sizing can be bypassed in certain defects with enough surrounding rims, it has traditionally been considered to be a crucial phase in the process. In fact, balloon sizing may offer additional information beyond the average defect size, such as the compliance of nearby rims and the occurrence of other defects.
While balloon stretched diameter and balloon occlusive diameter have previously been employed in balloon sizing, stop flow diameter (SFD) is now advised as the standard measurement to prevent oversizing. The suggested device size is the same as or slightly larger (by about 2 mm) than the SFD for self-centering devices such as the Amplatzer Septal Occluder (ASO) (St. Jude Medical, St. Paul, MN, USA). Nevertheless, the size of the device should be chosen individually taking into account the size of the heart, the spatial interaction with neighboring cardiac structures, and the lack of rims.
The standard advice is to steer clear of "oversized" devices in patients with aortic rim deficit due to the possibility of erosion. Use of an "undersized" device should be avoided in patients with inferior vena cava (IVC) rim insufficiency because they have a higher risk of device embolization. A device twice the size of the defect is advised when employing a non-self-centering device, such as the Gore Septal Occluder (GSO) (WL Gore & Associates, Inc., Flagstaff, AZ, USA), and the GSO is not advised for defects larger than 18 mm.
The use of specially designed devices to close the atrial septal defect (ASD) is an alternative to surgical closure of ASD. During cardiac catheterization, these devices are implanted utilizing a specialized delivery system through a catheter. This approach is thought to be less intrusive than open cardiac surgery.
Transcatheter device closure has the following benefits:
Prior to advising patients to use a transcatheter device to close their atrial septal defects, patients' conditions must be carefully evaluated. There are various devices available for ASD transcatheter closure.
Although it is done for a repairable hole and this is not the sole treatment for hole closures, the device closure process performed as a reparative surgery is known as transcatheter closure. It is a less intrusive surgery than other procedures.
DURING THE OPERATION:
An IV is inserted into the patient before surgery begins, and intravenous medication is started. The individual has ECG leads affixed to his chest. After cleaning the area around the person's groin and arm, a catheter will be put through it. The patient will be awake and conscious throughout the surgery while receiving sedatives for relaxation and local anesthetic.
In order to visualize the heart chambers and the size of the hole, a contrast dye would be injected into the arteries. An echocardiography or transoesophageal echocardiogram is also performed if needed.
Once the hole's size has been established, a special catheter that carries a tiny metallic mesh-like closure device is inserted into the arteries. Then, the catheter releases the gadget after it has been inserted into the hole. After the catheter is removed, the closure device eventually sprouts heart tissue and becomes an integral part of the heart.
POST-SURGERY:
When the catheter is removed, the region may need to be sutured or heavily bandaged. The patient spends one or two days in the hospital. A week after the doctor's recommendation, the patient can resume their normal routine. They are recommended to lead a healthy lifestyle that includes working out, eating well, and getting regular heart exams.
© Copyrights by Dr.Karthik Tummala 2022 Developed By KL ADS